Division of TB Elimination. 01 mL of 5 tuberculin units of PPD is injected intradermally on the volar aspect of the forearm which should raise a weal.

Tuberculin Purified Protein Derivative Injection Packaging Vial Rs 133 Pack Id 21985999562
PPD Tuberculin Skin Testing.

5 TUBERCULIN UNIT. TUBERCULIN PPD Purified Protein Derivative is used for the Tuberculin test. Do not inject intravenously intramuscularly or subcutaneously. Tubersol is indicated for intradermal injection only.
Results were read by trained staff 48-72 hours later. MEASUREMENTS AND MAIN RESULTS. Mantoux Tuberculin Skin Test.
From The Centers for Disease Control and Prevention. Administration examples below All tests should be read between 48 and 72 hours. Tuberculosis transmission along the US-Mexican border - 1990 Of the 160 clients and staff who were identified as contacts to the index patient 146 were tuberculin skin tested with 5 tuberculin units of purified protein derivative PPD using the Mantoux technique.
Five 5 tuberculin units TU per test dose of 01 mL is the standard strength used for intradermal Mantoux testing. TUBERCULIN PPD conforms to the requirements of the British Pharmacopoeia. 2 TU RT23 is commonly used in many countries and will result in smaller reactions.
An arbitrary unit of tuberculin dosage defined by comparison of clinical response to a preparation of the purified protein derivative standardized. TUBERSOL is indicated for intradermal injection only. Its potency is expressed in terms of the International Tuberculin Unit IU.
A Mantoux tuberculin skin test with 5 tuberculin units TU was positive 12 mm of induration. 5 TU is the norm in the US and results are read as induration not redness. Five tuberculin units of purified protein derivative PPD tuberculin are injected intradermally with the bevel of the needle pointing upward to form a wheel of 6 to 10 mm.
The tests most commonly used in Australia are the Mantoux test and the Heaf test. Do not inject intravenously intramuscularly or subcutaneously. Five 5 tuberculin units TU per test dose of 01 mL is the standard strength used for intradermal Mantoux testing.
Pain was assessed with a 10-unit numeric scale. The tuberculin is administered using a single-dose disposable tuberculin syringe that has a one-quarter to one-half inch 27-gauge needle with a short bevel. The injection should be made with a disposable 27-gauge tuberculin syringe just beneath the surface of the skin with the needle bevel facing upward.
If subcutaneous injection occurs the test cannot be interpreted. For example a reaction of 5mm in a child who is a contact of a person with smear-positive tuberculosis probably indicates tuberculous infection and is considered positive. Twenty four hours after placement of the skin test the maximum transverse diameter of induration was measured with a transparent plastic ruler by two of the authors.
If subcutaneous injection occurs the test cannot be interpreted. In some countries 15 mm is used if the patient had had BCG. In the United States the Mantoux tuberculin skin test consists of an intradermal injection of exactly one tenth of a milliliter mL which contains 5 tuberculin units.
A 5 tuberculin unit dose of PPD Tuberculin PPD InterVax Biologicals Limited Canada Serial No 3850196 was administered by a single experienced nurse using the Mantoux technique. Tuberculosis Screening in a Dialysis Program Testing was performed using five tuberculin units of purified protein derivative PPD tuberculin by the Mantoux method. Participating nurses used standard measuring tape to determine the largest diameter of each hematoma.
5 mm is positive for high risk situations close contacts HIV 10 mm is positive for most other situations. Reactions to the Mantoux test are interpreted on the basis of a quantitative measurement of the response to a specific dose 5 TU PPD-S or equivalent of Tuberculin. One group received enoxaparin injections with a 30-gauge 516-inch insulin syringe and the other group was injected with a 26-gauge 38-inch tuberculin syringe.
The 5TU dose of Tuberculin PPD intradermally Mantoux is indicated as an aid in the detection of infection with Mycobacterium tuberculosis. One unit 1 IU is equivalent. If more than 72 hours has elapsed and there is not an.
Five tuberculin units equivalent to 00001 mg of the international standard is the dose widely used for tuberculin skin testing. Likewise a 5-mm reaction in a person with known HIV infection should be considered positive. Skin testing should be performed by intradermal injection Mantoux method of 5 tuberculin units of antigen in 01 mL usually into the skin of the volar surface of the forearm.
Test AdministrationGive 01 ml of 5 Tuberculin Units PPD intradermally. The Mantoux tuberculin skin test TST is performed by placing an intradermal injection of 01 ml of purified protein derivative PPD containing 5 tuberculin units TU into the volar surface of the forearm.
Tempat Oleh Oleh Khas Balikpapan Mantoux Test

Tb Testing Current Thinking Ppt Video Online Download
Https Www1 Publichealthgreybruce On Ca Portals 0 Topics Infectiousdiseases Tuberculosisskintestmantouxtechnique Tb 20mantoux 20testing 20algorithm Pdf

Pdf Tuberculin Skin Testing Indication Interpretation And Management
Tuberculosis Tb Phcl 442 Lab Discussion 4 Raniah Al Jaizani M Sc Ppt Download

Tb Skin Testing Ppt Video Online Download

Aplisol Ppd 10 Test 5 Unit 0 1 Ml 1 Ml Vial 1 Bx

Tuberculosis Dr Awadh Alanazi 2015 1436 Objectives By
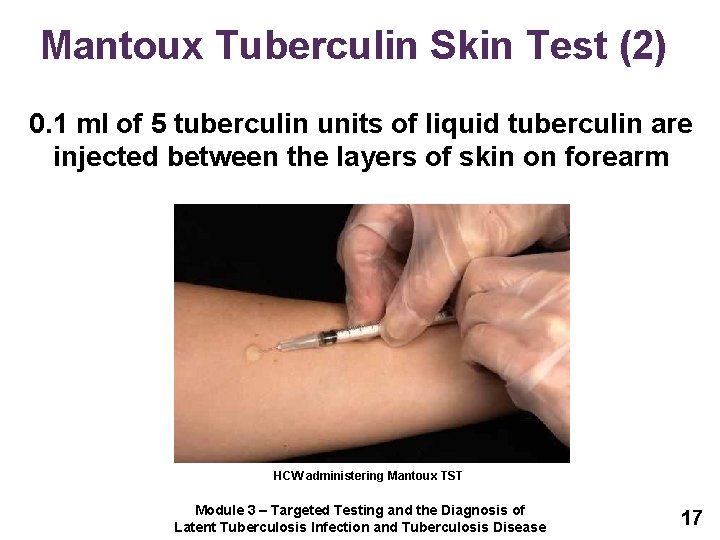
Selfstudy Modules On Tuberculosis Targeted Testing And The

Tuberculosis Dr Awadh Alanazi 2015 1436 Objectives By

Tubersol 5 Tu 0 1 Ml 1 Ml Multi Dose Vial 10 Test Pack

Six Simultaneous Tuberculin Skin Tests On The Same Person Slightly Download Scientific Diagram

Tes Mantoux Mantoux Test Pusat Imunisasi

Tuberculin Ppd Rt 23 Ssi For Mantoux Test

Guidelines For Administering The Mantoux Tuberculin Skin Test

Sanofi Pasteur 752 21 Mckesson Medical Surgical
Https Med Unhas Ac Id Kedokteran Wp Content Uploads 2017 09 Tuberkulin 2107 Pdf

Rx Item Tubersol 5tu 50test Vial 5 Ml By Sanofi Pasteur


